Covid Vaccine Long Term Side Effects Google Scholar
Articles theses books abstracts and court opinions. Emphasizing the haste of vaccine development the need for long-term safety data and concern that side effects could make everything worse than it.

Old Vaccines For New Infections Exploiting Innate Immunity To Control Covid 19 And Prevent Future Pandemics Pnas
Additional studies and long-term population-level surveillance are strongly encouraged to further define the safety profile of COVID-19 vaccines.

Covid vaccine long term side effects google scholar. Based on 29 included articles the most common reasons respondents stated they were hesitant to receive the COVID-19 vaccine included concerns about long-term side effects and results overall distrust of the manufacturer and the manufacturing process and skepticism regarding the efficacy of the vaccine against COVID-19. A sore arm from the injection. To provide comprehensive overview of the safety profile of COVID-19.
Or caused by new or relapsing inflammation ongoing infection or side-effects of immunomodulatory treatment. While the benefits of vaccination far outweigh the risks in the vast majority of cases it is prudent. 5 6 7 Over the past decades vaccine hesitancy has steadily grown partly due to fear of side effects arising from vaccination.
Pulmonary involvement during the acute disease. Research suggests that the willingness of people getting immunization for COVID varies from 55 to 90. We are writing to express concern about the use of a recombinant adenovirus type-5 Ad5 vector for a COVID-19 phase 1 vaccine study1 and subsequent advanced trials.
Weekly report updated to include data up to and including 20 October 2021. Weekly update to adverse reactions to COVID-19 vaccines. There is absolutely no evidence that covid-19 vaccines can affect the fertility of women or men says new expert guidance.
The guidance1 published by the Association of Reproductive and Clinical Scientists and the British Fertility Society comes amid concerns that misinformation that has been circulating online about covid-19 vaccines and fertility may be putting some women off having. Clinical trials of some vaccines involved more than 40000 participants and yielded few signs of side effects beyond those often seen after vaccination including injection-site soreness fever. The recent news of Pfizer and BioNTechs COVID-19 vaccine gave the world a sense of light at the end of a tunnel.
In this prospective observational study we examined the proportion and probability of self-reported systemic and local side-effects within 8 days of vaccination in individuals using the COVID Symptom Study app who received one or two doses of the BNT162b2 vaccine or one dose of the ChAdOx1 nCoV-19 vaccine. The short-term side effects of the authorized COVID-19 vaccines are similar. A small fraction of vaccinated persons nonetheless had more severe autoimmune manifestations following the first or second vaccines doses.
This will require the continuous monitoring of vaccinated patients for possible adverse reactions to COVID-19 vaccines. Based on these statements of hesitancy further research. You may also get a high temperature or feel hot or shivery 1 or 2 days after your vaccination.
The COVID-19 vaccines can cause mild adverse effects after the first or second dose. Like all medicines the COVID-19 vaccines can cause side effects but not everyone gets them. The occurrence of adverse effects is reported to be lower in the PfizerBioNTech vaccine compared to the.
If anything long-term side effects from the coronavirus spike protein and entire viral genome are more worrying than mRNA vaccines spike protein and fragile mRNA. Search across a wide variety of disciplines and sources. Google Scholar provides a simple way to broadly search for scholarly literature.
Over a decade ago we completed the Step and Phambili phase 2b studies that evaluated an Ad5 vectored HIV-1 vaccine administered in three immunisations for efficacy against HIV-1 acquisition23 Both international. As there is a lack of rigorous data from long-term trials on the safety of COVID-19 vaccines there is an urgent need to strengthen postmarketing surveillance of adverse event data particularly in low- and middle-income countries. Feeling or being sick.
Such data can serve to point at candidate management. This study aims to systematically review and synthesize the evidence on the safety data from the published COVID-19 vaccine. Also the vaccine is not 100 effective and has reduced efficacy if not stored at precise temperatures vaccinated subjects may.
Google Scholar 3. With the duration of the clinical trials as they are long-term side effects and whether the vaccine will provide lasting immunity are unknown. Most side effects have been mild and associated with short-term reactions to the vaccine including injection site reactions fever and flu-like symptoms.
Most side effects are mild and should not last longer than a week such as. The rapid process of research and development and lack of follow-up time post-vaccination aroused great public concern about the safety profile of COVID-19 vaccine candidates. The side effects typically start within a day or two of getting the vaccine and may include.
You can take painkillers such as. A two-dose regimen of BNT162b2 30 μg per dose given 21 days apart was found to be safe and 95 effective against Covid-19. Adoption of safety measures systematic.
However there is no comprehensive review of safety data reported from the vaccine trials which is critical information to inform the policies in order to improve uptake of COVID-19 vaccines and mitigate the risk aversion perceived due to the COVID-vaccine side effects. Long-term effects of coronavirus disease 2019 COVID-19. The COVID-19 vaccines can cause mild adverse effects after the first or second doses including pain redness or swelling at the site of vaccine shot fever fatigue headache muscle pain nausea vomiting itching chills and joint pain and can also rarely cause anaphylactic shock.
Some reports have shown the neurologic side effects after immunization mainly demyelinating diseases Table 1. 4 November 2021. We also compared infection rates in a subset of vaccinated individuals.
The main concern regarding vaccination in all groups proved to be the long-term side effects of the vaccine. If indeed COVID-19 is causing long-term sequelae then are the mechanisms underlying the long-term consequences immunological. In the previous study by this research group which was performed on medical and non-medical students the analogous questionnaire was available from 22 December 2020 to 25 December 2020.
The meta-analysis of the studies included an estimate for one symptom or more reported that.

Safety Of Covid 19 Vaccines Administered In The Eu Should We Be Concerned Sciencedirect

Efficacy And Safety Of The Mrna 1273 Sars Cov 2 Vaccine Nejm

Safety And Efficacy Of The Bnt162b2 Mrna Covid 19 Vaccine Nejm

Covid 19 Vaccination In Patients With Reported Allergic Reactions Updated Evidence And Suggested Approach The Journal Of Allergy And Clinical Immunology In Practice
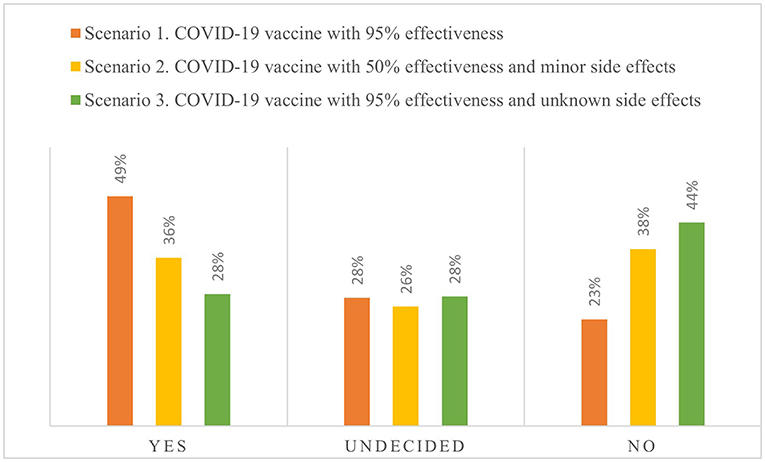
Frontiers Hesitation And Refusal Factors In Individuals Decision Making Processes Regarding A Coronavirus Disease 2019 Vaccination Public Health
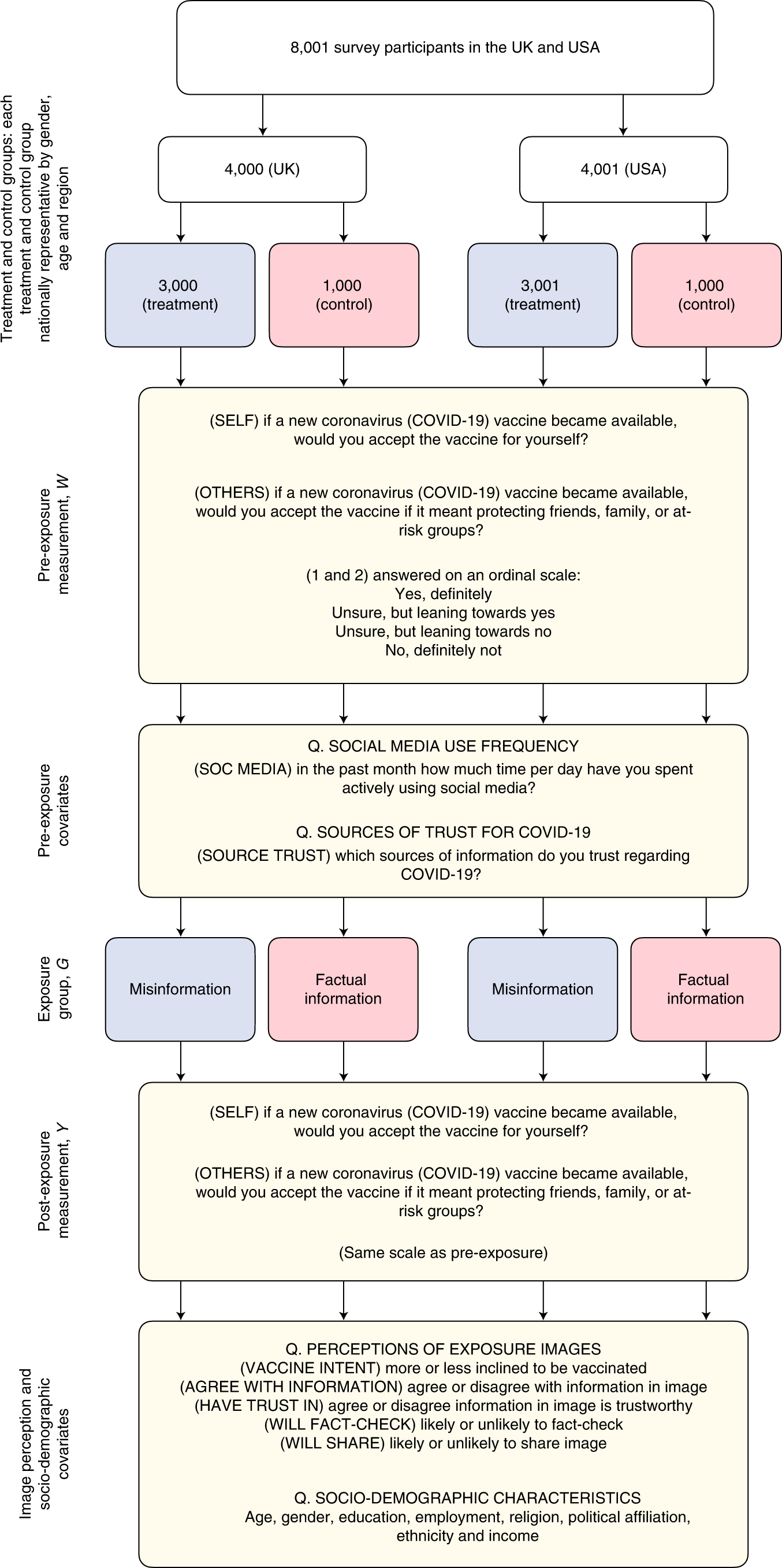
Measuring The Impact Of Covid 19 Vaccine Misinformation On Vaccination Intent In The Uk And Usa Nature Human Behaviour

Vaccine Side Effects And Sars Cov 2 Infection After Vaccination In Users Of The Covid Symptom Study App In The Uk A Prospective Observational Study The Lancet Infectious Diseases
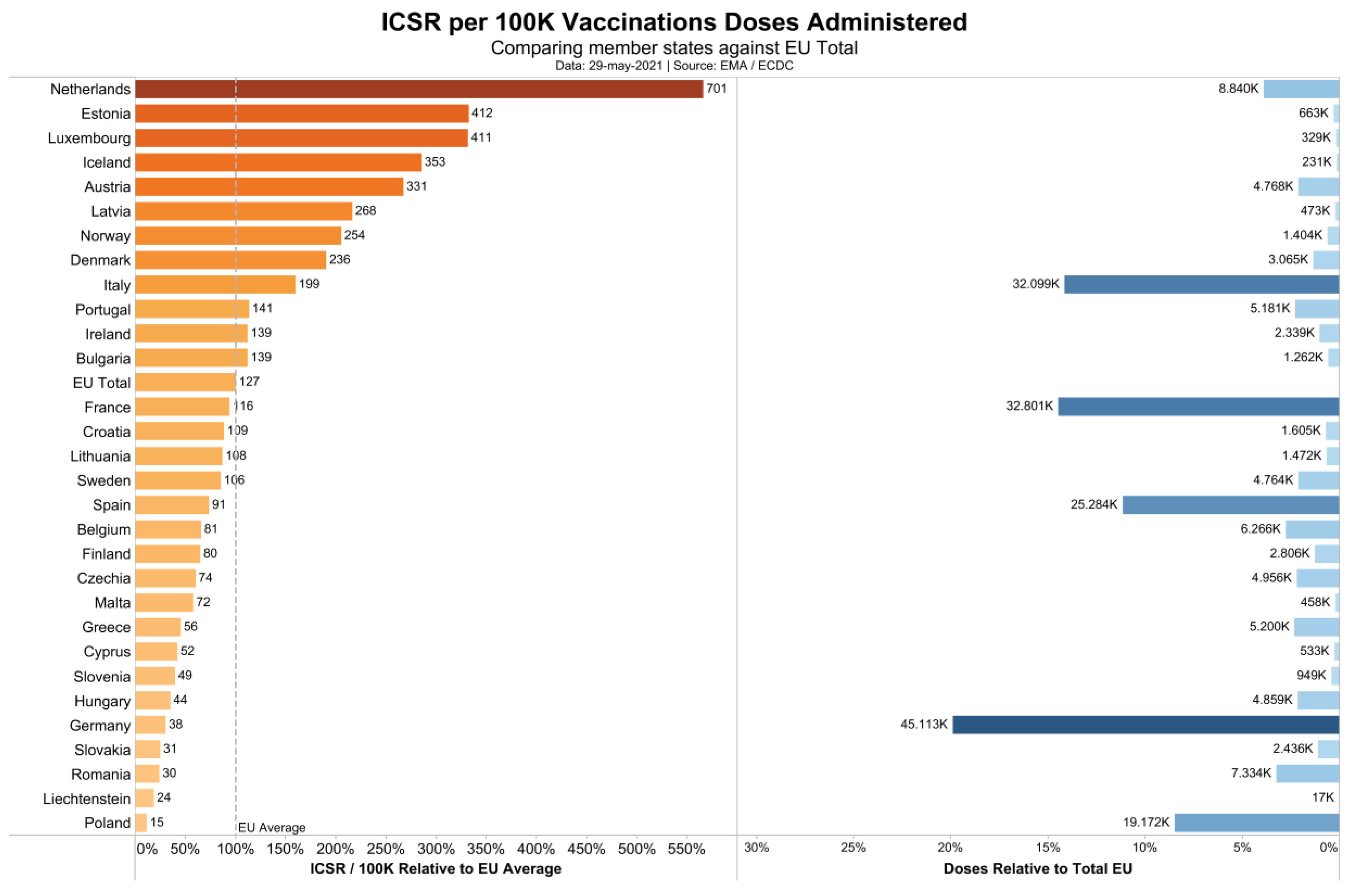
Vaccines Free Full Text The Safety Of Covid 19 Vaccinations We Should Rethink The Policy Html

Safety And Covid 19 Symptoms In Individuals Recently Vaccinated With Bcg A Retrospective Cohort Study Sciencedirect
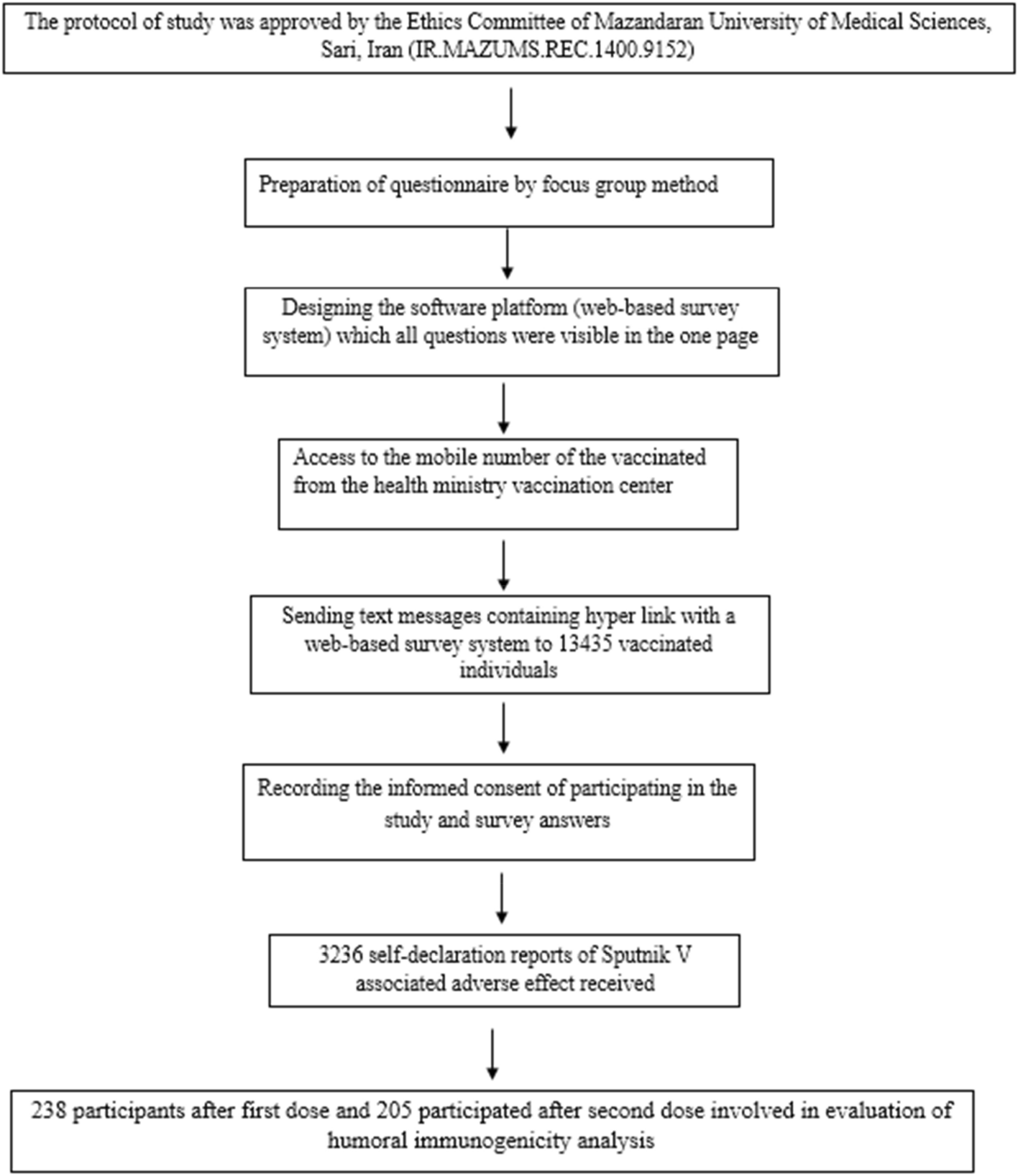
Side Effects And Immunogenicity Following Administration Of The Sputnik V Covid 19 Vaccine In Health Care Workers In Iran Scientific Reports

Vaccine Side Effects And Sars Cov 2 Infection After Vaccination In Users Of The Covid Symptom Study App In The Uk A Prospective Observational Study The Lancet Infectious Diseases

Safety Of Covid 19 Vaccines Administered In The Eu Should We Be Concerned Sciencedirect

Short Term Safety Of The Bnt162b2 Mrna Covid 19 Vaccine In Patients With Cancer Treated With Immune Checkpoint Inhibitors The Lancet Oncology

Interim Results Of A Phase 1 2a Trial Of Ad26 Cov2 S Covid 19 Vaccine Nejm
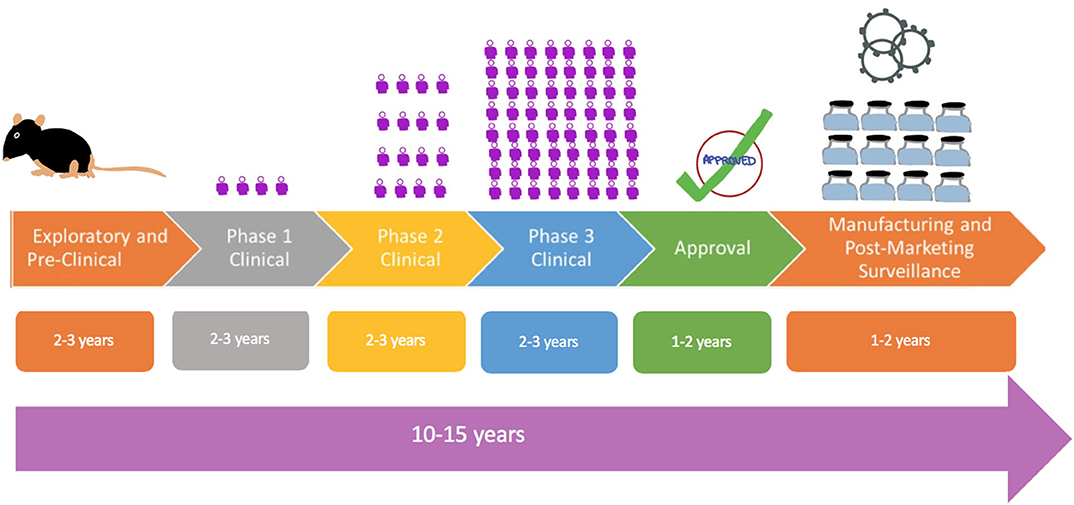
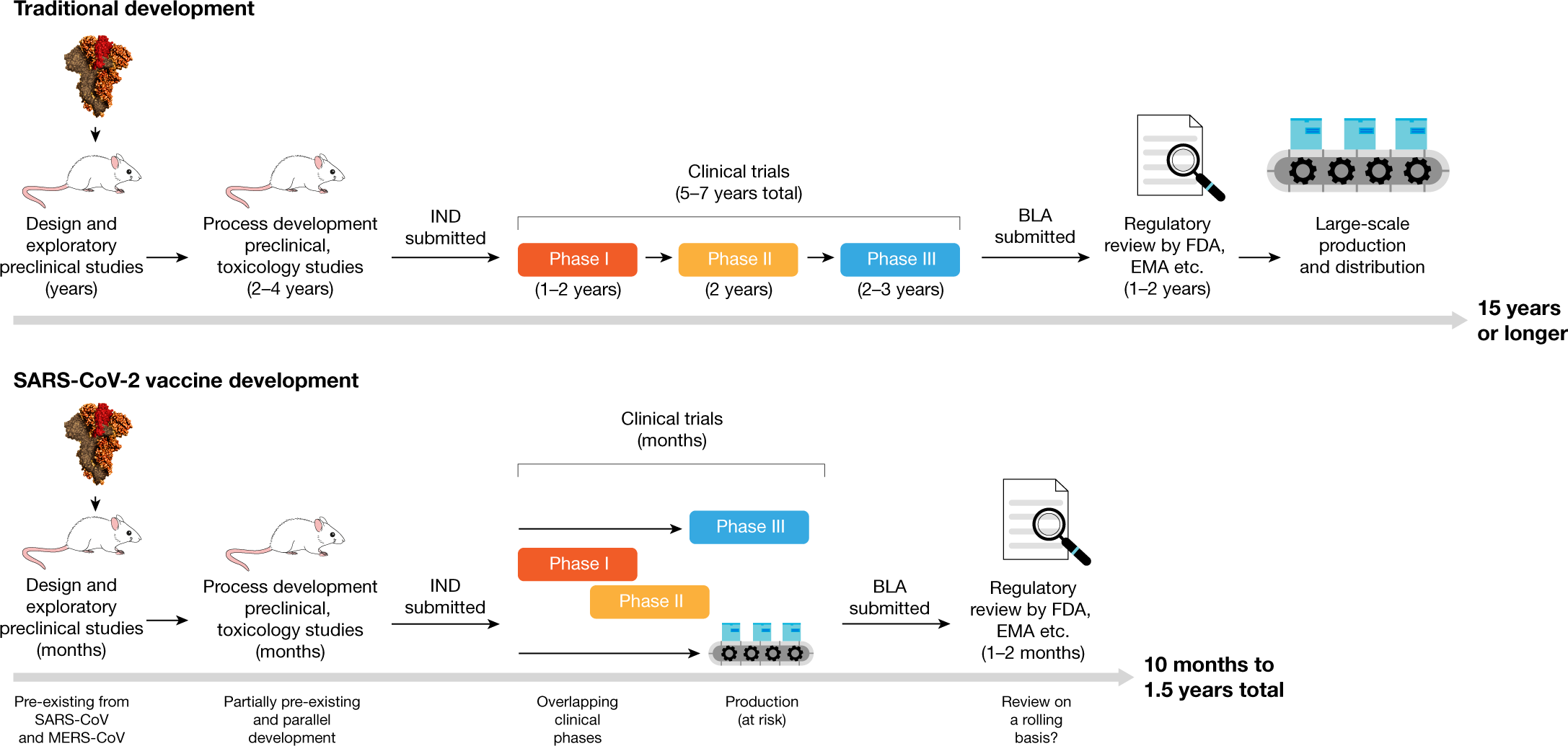
Frontiers A Review Of The Progress And Challenges Of Developing A Vaccine For Covid 19 Immunology

Safety And Efficacy Of The Chadox1 Ncov 19 Vaccine Azd1222 Against Sars Cov 2 An Interim Analysis Of Four Randomised Controlled Trials In Brazil South Africa And The Uk The Lancet
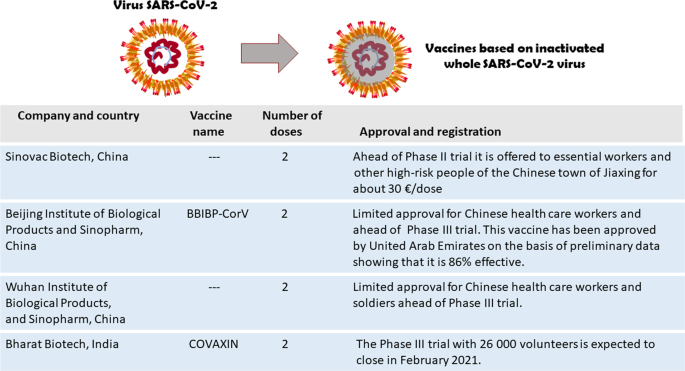
Covid 19 Vaccines Where We Stand And Challenges Ahead Cell Death Differentiation
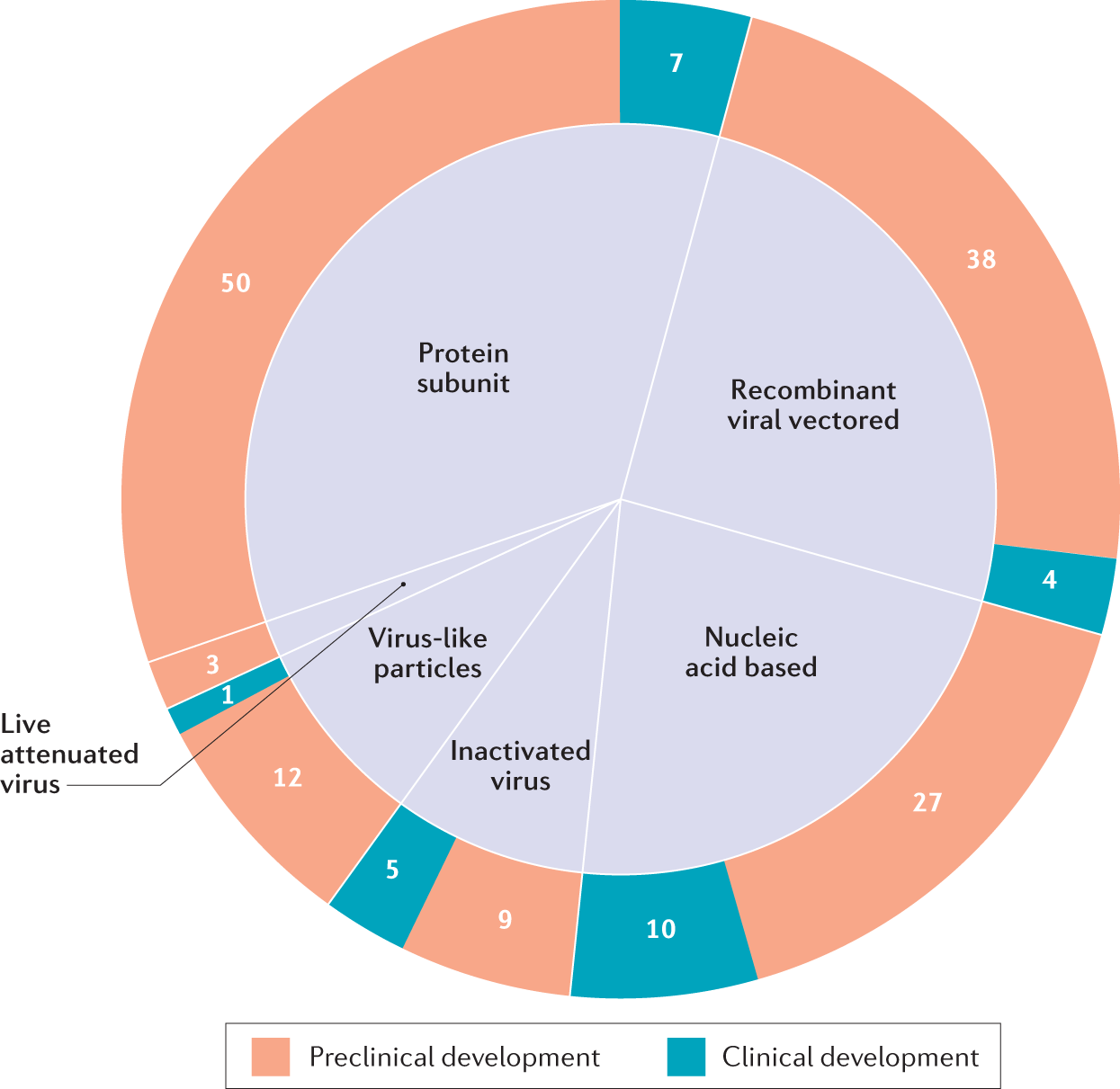
Immunological Considerations For Covid 19 Vaccine Strategies Nature Reviews Immunology